Summary:
- The PrEPVacc HIV vaccine trial in Masaka, Uganda, has been halted due to concerns about its efficacy, with the Independent Data Monitoring Committee finding insufficient evidence of the tested vaccines reducing the risk of HIV acquisition.
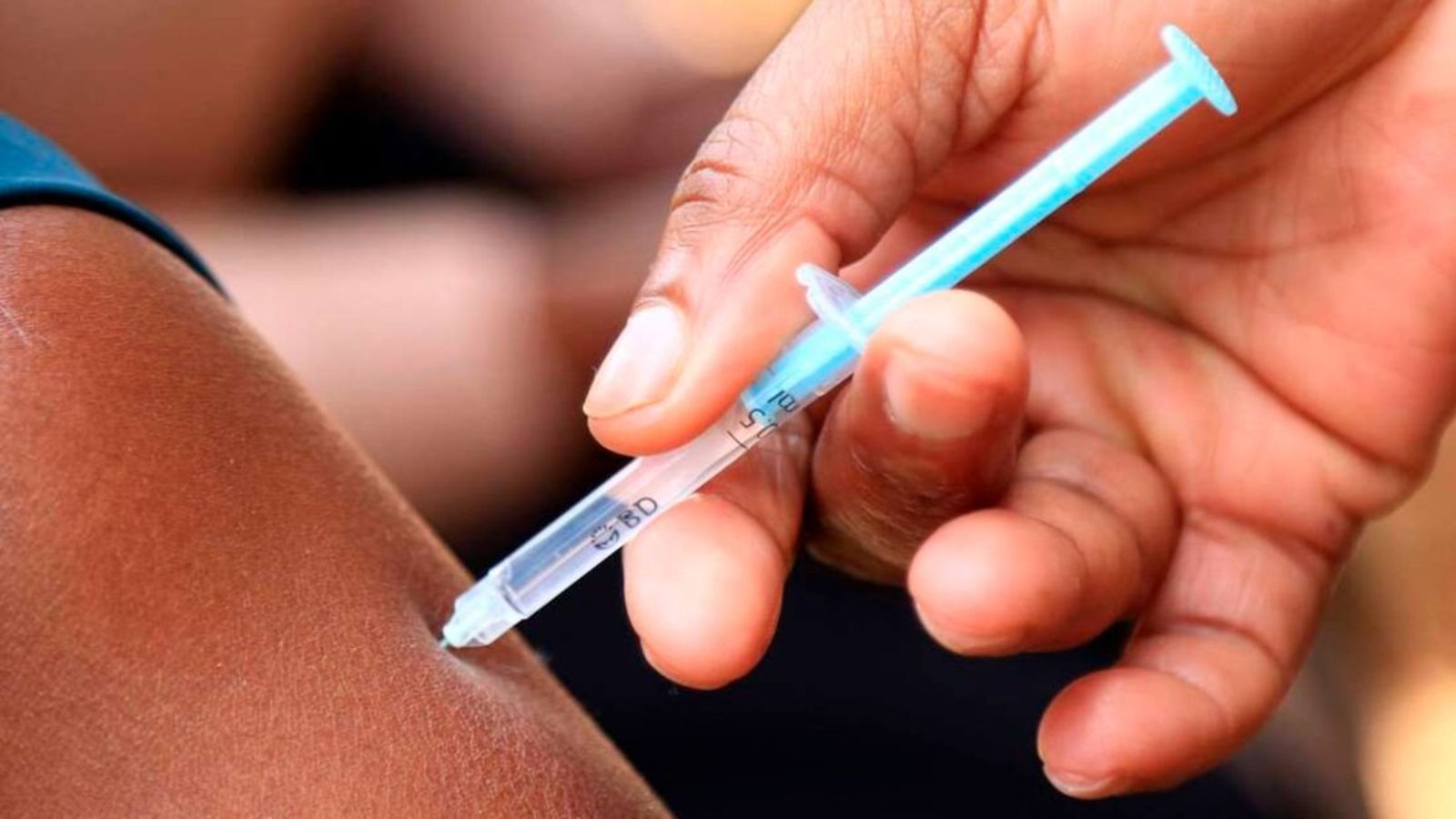
The PrEPVacc clinical trial, an experimental HIV vaccine study conducted in Masaka, Uganda, has been suspended due to concerns regarding its effectiveness in preventing HIV infections. Launched in December 2020, the trial aimed to assess the efficacy of experimental vaccine regimens and a novel form of oral pre-exposure prophylaxis (PrEP).
Dr. Eugene Ruzagira, the trial director affiliated with the Medical Research Council/Uganda Virus Research Institute and the London School of Hygiene and Tropical Medicine (MRC/UVRI & LSHTM) Uganda Research Unit, revealed that the Independent Data Monitoring Committee scrutinized the collected data. Their analysis indicated insufficient evidence to support the notion that the tested vaccines effectively reduce the risk of acquiring HIV. Consequently, vaccinations for participants in the PrEPVacc trial have been halted.
The PrEPVacc initiative, led by African researchers in Entebbe, Uganda, and sponsored by Imperial College London with support from 15 partner organizations across Africa, Europe, and the US, faced this setback. Despite the disappointment, Prof Pontiano Kaleebu, the Chief Investigator of PrEPVacc, underscored the ongoing importance of developing an HIV vaccine tailored for Africa.
Let Us Build Your Online Success!
We are the experts in creating visually stunning and functional websites. With reliable hosting and exceptional customer support, we bring your vision to life. Join hundreds of happy clients who trust us!
Get Started Now📞 Call/WhatsApp: +256 207 800 192
The vaccine’s failure comes three decades after a prior trial for an experimental HIV prevention vaccine in Uganda. The current trial utilized an experimental vaccine candidate provided by the Switzerland-based EuroVacc Foundation.
Uganda, like other nations globally, aspires to eradicate AIDS by 2030, with preventing new infections being a pivotal objective. Although the vaccine trial has been paused, the follow-up of participants will persist for additional safety data collection, HIV testing, and referral for ongoing care. This monitoring will extend for six months after the last vaccine injection or until the conclusion of the oral PrEP trial, depending on which timeframe is longer. The independent committee recommended the oral PrEP component of the study to continue until completion.